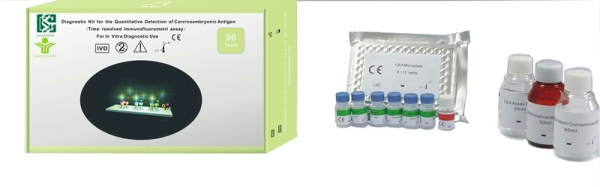
【INTENDED USE】
The Diagnostic Kit for the Quantitative Detection of Carcinoembryonic Antigen (Time-resolved immunofluorometric assay) is a solid phase, two-site fluoroimmunometric assay for the quantitative detection of carcinoembryonic antigen in human serum.
【SPECIFIC PERFORMANCE CHARACTERISTICS】
1. Sensitivity
1107 samples (457 abnormal, 650 normal) were collected and tested. The result shows that the minimum detectable concentration of CEA by this assay is less than 0.5 ng/ml.
2. Dosage-reaction curve linea(r) should be more than 0.9900.
3. Repeatability
The inner analysis precision: CV%10.0; the inter analysis precision: CV%15.0
4. Specificity
The test result of AFP(1000 U/ml), CA125(600 U/ml), CA19-9(500 U/ml) and albumin(1000 ng/ml) detected by the kit is lower than 0.15 ng/ml, 0.15 ng/ml, 0.15 ng/ml and 0.20 ng/ml.
5. Anti-interference
The assay is not interfered by Triglyceride(21.54 mmol/L), bilirubin(818µ mmol/L) and hemoglobin(18 g/L) Measuring range The measuring range of the kit is from 0.02 ng/ml to 830 ng/ml.
 Bstar300 superconductive 3.0T
Bstar300 superconductive 3.0T Gynecology operation bed
Gynecology operation bed Bladdar Scanner
Bladdar Scanner Raecho Laptop Color Doppler
Raecho Laptop Color Doppler Lower Limbs Instrument
Lower Limbs Instrument Upper Limbs Instrument
Upper Limbs Instrument Female sterilization surgery
Female sterilization surgery Ophthalmic Surgical Instrument Package
Ophthalmic Surgical Instrument Package DC-N2 Color Doppler
DC-N2 Color Doppler S30 Color Doppler Ultrasound
S30 Color Doppler Ultrasound S40 Color Doppler Ultrasound
S40 Color Doppler Ultrasound BC5150 Hematology Analyzer
BC5150 Hematology Analyzer DC-70 Color Doppler Ultrasound
DC-70 Color Doppler Ultrasound Ebola Protection Clothes
Ebola Protection Clothes DC-7 Echocardiograph
DC-7 Echocardiograph Medical Incinerator
Medical Incinerator PLX-101D Radiology
PLX-101D Radiology E30 Color Doppler
E30 Color Doppler Qbit9 Supreme
Qbit9 Supreme Qbit 7 Color Doppler
Qbit 7 Color Doppler MRI system 0.4t
MRI system 0.4t