Product Center
- Radiology
- MRI System
- Angiography Intervention System
- Ultrasound Biospy Bracket
- Veterinary Ultrasound
- Protective Lead Screen
- C-Arm X Ray system
- Black/White Ultrasound
- Digital Mammography
- Mammography Non Digital
- Automatic Film Processor
- Digital X-Ray DR
- Fluoroscopy
- Color Doppler Ultrasound
- CT
- Protective Collar
- Arms/Hands Protective
- Lead Apron
- Intervention Radiation Protective Gloves
- Portable X-ray unit
- Panoramic Dental X ray unit
- X-Ray system
- Protective Lead Glass
- Standing X-ray Film Cassette Shelf
- Photographic Studio
- Fluroscopic Room
- Lead Defense Chair
- Film Storing Box
- Apron Rack
- Darkroom Light
- Film Developping Tank
- Mobile X-ray
- Developping Hanger
- Integrated Information System
- X-Ray Dosimeter
- Film Cassettes
- Protective Glasses
- Protective Mask
- Children Protection
- Protective Scarf
- High Light Viewer
- Protective Cap
- X ray Film
- OB/GYN
- Postpartum Rehabilitation Instrument
- Intervention Red Light Treatment
- Color Ultrasound
- Doppler
- Fetal Monitor
- U.V Sterilizer
- Side Lamp
- Digital Colposcope System (Colpovision)
- Operation-Examining Bed
- Mayo Stand
- Dressing Car
- Kick Bucket
- Laparoscopic system
- Anorectal Treating Device
- OBGYN Instrument package
- Inhalation Analgesia Device
- Breast Therapy Instrument
- Disposable Dilator
- Leep Electro surgical unit
- Medical Washing Machine
- Microwave therapy equipment
- Cold Treatment equipment
- Abortion system
- Semi conductor laser machine
- Ultrasonic treatment apparatus
- Cervical Treatment Instrument
- Pelvic inflammatory
- Disposable
- Examination Gloves
- Cuvette
- Biplatrix
- Jar Redon
- CERVICAL COLLAR HARD
- COMPRESSES 40 X 40
- Drain Redon
- FILS NYLON
- Medical Examination Drape
- GANTS STERILES(Surgica Gloves)
- First Aid Kit
- JERSEY TUBE
- BLADE KNIFE
- Needles
- Soft Bandage
- Adhesive tape perforated
- TULLE GRAS
- VICRYL(synthétic absorbable)
- Glove clean unit
- Bedpan With Plastic Handle
- Specimen cup
- Test Tube Rack
- Centrifuge Tube
- Vaccin Specimen Tube
- Petri Dish
- Test Tube
- Swab
- Crepe tape
- Tip
- Filter Cone
- Umbilical Clamp
- Identification Bracelet (insert card)
- Vaginal Speculum
- Condom Catheter
- Stick
- Crutch
- Folding Stick
- Straw
- Ostomy
- Disposable Connecting Grain
- Disposable Anesthesia Puncture Package
- Disposable Stomach Tube
- Disposable Feeding Tube
- Disposable Operation Package
- Disposable Maternity Package
- Disposable Catheterization Package
- Synthétic absorbable
- Disposable Angiography Surgical Kits
- Disposable Dressing Package
- Urostomy
- Height Scale
- Tongue Depressor
- BP Monitor Package
- Thermometer
- Test Rapid
- Dust-Proof Face Mask
- Disposable Sunction Tube
- Tourniquet
- Scale
- Skirt has 2mL cryovials tubes Boucho Screwing Away
- Vacuum blood collection tubes ss 4-5 ml red / dry
- Vacuum blood collection tubes ss 4-5 ml purple
- Vacuum blood collection tubes ss 4-5 ml Grey
- Reagent
- Otoscope
- Disposable Connecting Pipe
- Sphygmomanometer
- Disposable Dental Package
- Reflex Hammer
- Pipette
- Ear Tip
- Name Tag
- Penlight
- Disposable Catheter
- Disposable Anal Caral
- 15ml polypropylene Falcon tube
- Sthetoscope
- Operation Room
- General Surgery-Minor Surgery Set
- Kick Bucket
- Mayo stand
- Scrub Station
- Suction
- Electrosurgical Unit-Electrotome
- Syringe Pump
- Infusion Pump
- Instrument Package
- Anesthesia Machine
- Dressing Car
- General Surgery-Laparatomy Set
- Examination Lamp
- General Surgery-C/S Set
- Autoclave
- Surgical Operation Lamp
- Electric Operating Table
- Oprating Table
- Patient Monitor
- Patient Monitor(with ETCO2)
- Electric Tourniquet
- Examination Table
- Anesthesia disposable
- Magnifier
- Orthopedics Frame
- Manual Blood Collection Chair
- Electric Blood Collection Chair
- Operation Instruments
- Defibrillator
- I.V STAND (Moving Type)
- Pulse Oximeter
- Head light
- Cast Cutter
- Operating Microscope
- Patient Stool
- Steps Still
- Stretcher Car
- Instrumental Cabinet
- Instrumental Table
- Medical Pendant
- Fetal Monitor
- Laboratory
- Coagulation Analyzer
- Magnetic and Electric Stirrer
- Roller Mixer
- Laminar Flow Cabinet
- Rotator (Slide Shaker)
- Incubator
- Water Purification System
- Blood Bank Refrigerator
- Haemocytometer
- Water Bath Thermostatic
- Hemoglobin Machine
- Timer
- ESR Analyzer
- Electrolyte Instrument
- Blood Culture Analyzer
- Micropipets of Dierent Size
- Water Distillation Apparatus
- Refregerator, Deep Freezer
- Biological Safty Cabinet
- High Speed Centrifuge
- Hematocrit Centrifuge
- Biological Microscope
- Glucometer
- Urine Analyzer
- Biochemistry Analyzer
- Hematology Analyzer
- Erythrocyte Sedimetation Rate Instrument
- Baking Machine
- ELISA Analyzer
- Medical Hand Sink
- Special Centrifuge
- Low Speed Freeze Centrifuge
- High Speed Freeze Centrifuge
- Low Speed Centrifuge
- Nucleic Acid Extractor
- Portable PH meter
- Viscosity cup
- Spectrophotometer
- Bacti-Cinerator
- Tissue Dehydrating Machine
- Microplate Washer
- Microtome
- Blood rheology analyzer
- Immunoassay Analyzer
- PCR instrument
- Microplate Reader
- Agricultural Instruments
- Blood Gas Analyzer
- Fecal Occult Blood Analyzer
- Dry Oven
- Auto Microbial ID/AST Analyzer
- Reagent (reactif)
- Balance
- POCT Analyzer
- Orthopedics
- ENT
- Emergency Room
- Mayo Stand
- Emergency trolley with C.P.R Cart
- Intubation Set
- Defibrillator
- Infusion pump
- Syringe pump
- Side Lamp
- Suction
- E.K.C (Cart+Hanger/With program CD)
- Kick Bucket
- Vital signs monitor (rolling stand)
- Dressing Car
- Ambulance
- Patient bed
- Patient Stool
- Instrument Storage Cabinet
- Stretcher Car
- Coaches(Ambulance)
- NG Tubes
- Lung Ventilator(M.F)
- Emergency Portable Ventilator
- Ambulance package
- U.V Sterilizer
- Delivery Room
- Infant Incubator
- Anesthesia Machine
- Infusion Pump
- Syringe Pump
- Baby Scale
- Instrumental Table
- Neonatal Bed
- Infant Warmmer
- Electrosurgical Unit
- Suction Machine
- Side Lamp
- Instrument Cabinet
- Mayo Stand
- Delivery Tables
- Infant Bath
- Doctor Stool
- Breast Pump
- I.V STAND (Moving Type)
- Stretcher Car
- Steps Still(2steps)
- Patient Bed(Manual Bed Double Crank)
- Folding Screen Stainless Steel
- Fetal Monitor
- Fetal Doppler
- Vital Signs Monitor
- Defibrillator
- Operating Lamp
- Exmaination Lamp
- Vaccum Extraction, Manual
- Physiotherapy
- Transcranial riagnetic treatment
- Traction Bed
- Electronic Acupunctoscope
- Electrotherapy Instrument
- Therapy Hyperosteogeny Instrument
- Pain Treatment Apparatus
- High Frequency Instrument
- Diathermy
- Rheumatism Treatment Instrument
- Computer Medium Frequency Electroth
- Portable Slope Board
- Brain recover unit
- Blind Way
- Inflammatory treatment
- Computer electro-yee treatment
- Treating light
- Nerve injury treatment
- Osteoporosis Therapy
- Brain health unit
- Computer Audio Medicine Therapy
- Air Pressure Wave Treatment
- Balance Function Evaluation
- Thermal Magnetic Resonance Treatment
- Computer Bone Treatment Instrument
- Seat Bicycle
- I.C.T
- TENS
- Ultrasound
- Paraffin Bath
- Infra Red
- Laser Therapy
- Microwave Diathermy
- Therapy Bed (P.T Bed)
- Dressing Car
- Toilet Armrest
- Stationary Bicycle
- Hemorrhoids Treatment Machine
- Cuff Weight & Cart set
- Dumbell & Cart set
- Gymnic Ball
- Electric Tilting Table
- Multi Exerciser (COMB E.X)
- C.P.M (Knee)
- Assistant Walker
- Multi-Purpose Treatment Bed
- Traction Chair
- Vertical Lift-down Table
- Rehabilitation Treadmill
- Wheel Chair
- Parallel Poles
- Blind Rod
- Crutch
- Shift Machine
- Protection Belt
- Stool
- Prevent Sprinking Bowl
- Patient Bed Dinner Table
- Language Practice Card
- Child SPA
- Commode Wheel Chair
- Weight Loss Practice Unit
- Shower Chair
- Angle-Joint Recover Unit
- Hearing Aid
- Finger Training Apparatus
- Manual Tilting Table
- Training Bed
- Bound Bed
- Mobile Bed
- Inspection Bed
- Neck Support
- Kitchenware for Training
- Chest Back Adjustment
- Lumbar Support
- Digital Channels Medicated Treatment
- Ultraviolet Therapy
- Miriam Wax Treatment
- Sputum Extraction Machine
- TDP Treatment Unit
- Hemiplegia Recover Unit
- Relax Massage Unit
- Boating Sport Unit
- Abdominal Muscle Training Unit
- Auxiltary Walk Practice Unit
- Elastic Tie
- Ladder Practice
- Soft Mattress
- Sand Bags
- Rib Wood
- Basketball Frame Unit
- Adjust Mirror
- Multifunction Practice Unit
- Entry Wall Control Force Unit
- Hand Support Unit
- Run Machine
- 3D Automatic Interference Wave
- Limbs Training Unit
- The baby room
- The oxygen chamber
- ICU
- Endoscopy room
- Burn treatment room
- Ward
- Food Car (30 Person)
- Patient bed
- Patient bed (electrical bed double crank)
- Patient bed (electrical bed with orthopedices)
- Bedside Cabinets (General)
- Over bed table
- Stainless steel folding screen
- Wheel Chair
- WALKER
- I.V STAND (Moving Type)
- Dressing Car
- Patient bed (manual bed double crank)
- Laundry Cart
- Urinal Male
- Stretcher Car
- Diagnostic Set
- Resuscitation Set
- Electronice Stove(2 Plates)
- Medication Cup Board
- Bed Pan Rack
- Wash Basin
- Wash Basin Stand(double)
- Bed Pan Adult
- Bed Pan Child
- Urinal Female
- Treatment Carriage
- Disinfection room
- Pulse Vacuum Table
- U.V Sterilizer
- Ultrasonic Cleaner
- Rock Drying Glove
- Dressing Car
- Instrument Cabinet
- Portable Sterilizer
- Table model sterilizer
- Vertical Autoclave
- Sterilizer
- Dry Heat Type
- Ozone Disinfection
- Pulse Vacuum Horizontal Autoclave
- Cassette Type
- UV Sterilizer
- Medical Washing Machine
- Water Purifier
- Thermostatic Bath
- Medical Hand Sink
- Plasma Sterilizer
- Horizontal Cylindrical Autoclave
- Cardiology
- Dental
- High Speed Tungsten Carbide Burs
- Dental Unit Complete
- Cement Spatulas
- Mouth Mirror
- Forceps (For Kid)
- Forceps (For Adult)
- Root Elevator
- Root Elevators Type T
- Rubber Polishing
- Brush Polishing
- Excavators
- Low Speed Tungsten Carbide Burs
- Root-Canal Plugger
- Medical Refrigerator
- Instrument Cabinet
- Dressing Car
- Patient Stool
- Panoramic X-Ray Unit
- Steam Sterilizer (Dental Autoclave)
- Amalgam Mixer
- Dental X-Ray
- Digital Dental X-Ray
- Sensing Dental Light
- Hard Anhydrite
- Vacuum Sterilizer 16L
- Oral Anesthetic Injector
- Dental Suction
- Dry Oven
- Stainless Steel Dental Plate
- Stainless Impression Tray / Tooth Underlay
- Micro Absorption Dryer
- Mouth Lamp Bult (wireless)
- Mouth Lamp Bulb (wire)
- Cotton&Dressing pliers
- Silicone Rubber Conveyor Gun
- Thermostatic Bath
- Dental Plaster
- Excavator
- Bunishers
- Plaster Spatulas
- Mallet
- Bone Chisels
- Crown Remover
- Agar Impression Material Syringue
- Aspirating Syringe
- Dental X-Ray Film
- Eye
- Slit Lamp Microscope
- Halogen Ophthalmoscope
- Trial Lease In Sets
- Eye Instrument Set
- Instrument Cabinet
- Retinoscope
- Phaco Emulsifier
- Cornea pachymeter
- Ultrasonic A/P Biometer
- Ultrasonic A/B Scanner
- Portable Ultrasound Biomicroscope
- Operation Microscope
- YAG Laser
- Femtosecond laser
- Image Capture System
- Digital Slim Lamp Microscope
- LED Vision Chart
- Portable Slit Lamp
- Stainless Steel Scissor
- Ophthalmic table
- Refractometer
- Chart projector
- Lens Edger
- Lensmeter
- Ultrasonic cleaning machine
- Auto Perimeter
- Vaccin
- Health Examination Center
- Outpatient Department
- Otoscope
- Strecher Mobile Type
- Stethoscope
- Sphygmomanometer
- Digital thermometer
- Oral thermometer
- Rectal thermometer
- Axillary thermometer
- Infant Scale
- Examination Coach
- Ophthalmoscope
- Electrocardiography-03Channel
- Reflex Hammer
- Refrigerator
- Dressing Instuments Sets
- Examnination Lamp
- Cabinet to keep Instruments and Medication
- Folding Screen
- X-Ray Film Viewer
- Enema Set
- Wheelchair
- Adult Scale
- Beauty Center
- Magnetic Wave Spot Instrument
- Cell Activation Instrument
- Laser machine of moles removal
- Digital Microcrystaline Dispel Scar
- Automatic Beautiful Skin System
- Microwave Treatment Machine
- Laser Wash Eyebrow
- Laser Pigment Treatment
- Laser Treatment Apparatus
- Fat Suction Machine
- Photon Hairdressing
- Laser Depilating Machine
- Deeper Liposuction Machine
- Video beam Laser
- Light Power Instrument
- Gathered Energy Liposuction
- Semiconductor Laser Treatment
- E Light Beauty Device
- Electric Fat Suction Machine
- Liposuction Equipment
- CO2 Laser Instrument
- Rejuvenation face-lift system
- Resonance Liposuction
- Delux Magic Fiber Body Compartment
- No Needle Beauty Machine
- Sun Bathing Machine
- Blood Gas Circle Instrument
- Spectrum Phototherapy Burn Fat
- Cosmetology Chair
- Beauty Bed
- Towel Alexipharmic Ark
- Ovarian Keep Fit Instrument
- Lymphdrainage Instrument
- Beauty Machine
- Living Cells Rebirth Skin Membrance Cultivating
- Automatic Slimming Machine
- SPA Sauna Room
- Multifunction Hydro Therapy Massage Bed
- Almighty Oxygen Jet
- Multifunction Breast Enhancement
- Computer Vichy Shower
- Mesotherapy
- Super Energy Resonance
- Resist Compression Metalbolization Balane Meter
- Dipping Tank-Type Aroma
- Lumbar Kidney Keep Fit
- Air Pressure Detoxification
- General Department
- Refrigeration Equipment
- Cabinet-Bed table
- Baby Trolley
- Child Care Bed
- Manual Care Bed
- Electric Care Bed
- Grinder
- Pill Making Machine
- Corpse Reverence
- Corpse Cold Storage
- Blood Bank Refrigerator
- Patient Record Trolley
- Medical Low Temperature Freezer
- Stainless Steel Thermos
- Dialysate Powder
- Oxygen Generator
- Nutrition Pump
- Infusion Pump
- Syringe Pump
- Blood Perfusion Machine
- Blood Dialysis Machine
- Model
- CPR Training Manikin
- Transcranial Doppler
- Oxygen Cylinder
- Pharmaceutical Refrigerator
- Stainless Steel Wastebin
- Stainless Steel Forcep
- Stainless Steel Rescue Trolley
- Stainless Steel Dressing Bowl
- Stainless Steel Dressing Frame
- Stainless Steel Dressing Trolley
- Thyroid Retractor
- Stainless Steel Care Trolley
- Stainless Steel Enema bucket
- Stainless Steel Workstation
- Stainless Steel Anoscope
- Stainless Steel Gyn examination bed
- Anesthesia Cart
- Stainless Steel Orthopedics 3 Crank Bed
- Stainless Steel Bottle
- Stainless Steel Square Plate Bracket
- Stainless Steel Square plate
- Stainless Steel Messtin
- Stainless Steel Bed Pan
- Stainless Steel Cabinet
- Stainless Steel Room-Check Trolley
- Side Sitting Injection Chair
- Stainless Steel Trolley
- Stainless Steel Patient dinning table
- Stainless Steel Nasoscope
- Stainless Steel Medicine Taking Cup
- Incinerator
- General Department II
- Ground Trolley
- Automatic Cleaning Machine
- Vacuum Flask Carrying Cart
- Bucket
- Transfusion Trolley/Chair
- Tubal Extracting Hook
- Gloves Shelf
- Chair in OR
- Reflector Lamp
- Test tube rack
- Tongue Forcep
- Gauze Jar
- Chromosome Frame
- Debridement Cart
- Feculeney Cart
- Finger Board (Ligation)
- Multi Function Opener
- Measuring Cup
- Stainless Steel Cup
- Sterilize Box
- Basin Frame
- Instrument Cabinet / Trolley
- Retractor(Ligation)
- Folding Screen
- Tower Clip
- Hand Immersion Bucket
- Chaperone Chair
- Dish Sleeve
- Mixing Tank
- Stainless Steel Asepsis Cart
- Tweezers Tube
- Treatment Plate
- Contraceptive Ring Fitting-Removing
- Dissecting Device
- Pliers isolated vas deferens
- Ligation Pack Man/Woman
- Induced Abortion Product
- Operation Instrument Bag
- Cervical Dilator
- Uterine Stylet
- Mattress
- Stretcher
- Uterine Curette
- Collecting Tank
- Boiler
- Stainless Steel Step Desk
- Medicine Spoon
- Medicine Preparing Cabinet
- Clean Basin
- Stainless Steel Sterile Bottle
- Urinal Hand Carry
- Intestinal Spatula
- Chinese Medicine Dipper
- Kidney Dish
- Chinese Medicine Cabinet
- Ointment Jar
- Vaginoscope
- Vaginal Retractor
- Baby Height Measuring
- Syringe Needle Sterilizer
- Maternity Bag
- Tongue Spatula
- Urology
- Stone section
- Raecho Principal Products
- Mindray Gallery
- Ebora virus Protection
- Devices of Sleep Surgery
- Hemodialysis Room
- Raecho Ultrasound
- Tumor Treatment (HIFU)
- GE Gallary
- Philips Ultrasound
- Neusoft Galerie
- Siemens Gallery
- Emperor Ultrasound
- DAAN Gene Collection
- Endoscopy High-End
- High End Lancet
- Scaple High End
- Mom & Baby
- Infusion Management
- Solar Water Pump
- Laboratory
|
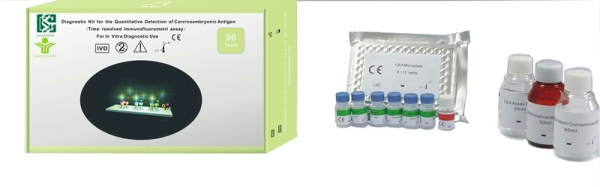
products name:
Kit Detection of Carcinoembryonic Antigen
|
|
|
【INTENDED USE】 The Diagnostic Kit for the Quantitative Detection of Carcinoembryonic Antigen (Time-resolved immunofluorometric assay) is a solid phase, two-site fluoroimmunometric assay for the quantitative detection of carcinoembryonic antigen in human serum.
【SPECIFIC PERFORMANCE CHARACTERISTICS】 1. Sensitivity 1107 samples (457 abnormal, 650 normal) were collected and tested. The result shows that the minimum detectable concentration of CEA by this assay is less than 0.5 ng/ml. 2. Dosage-reaction curve linea(r) should be more than 0.9900. 3. Repeatability The inner analysis precision: CV%10.0; the inter analysis precision: CV%15.0 4. Specificity The test result of AFP(1000 U/ml), CA125(600 U/ml), CA19-9(500 U/ml) and albumin(1000 ng/ml) detected by the kit is lower than 0.15 ng/ml, 0.15 ng/ml, 0.15 ng/ml and 0.20 ng/ml. 5. Anti-interference The assay is not interfered by Triglyceride(21.54 mmol/L), bilirubin(818µ mmol/L) and hemoglobin(18 g/L) Measuring range The measuring range of the kit is from 0.02 ng/ml to 830 ng/ml. Add to Enquiry List |
|